Describe the Composition of an Atom
A 250-cm-long cylindrical glass tube sealed at one end is filled with ethanol. An extremely small amount of a thing or quality.
Carbon Atom What is it.

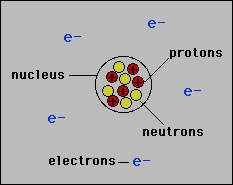
. A It is composed of protons. The electrons of the atom revolve around the nucleus in definite circular paths known as orbits which are. The protons and neutrons are at the center of the atom and make up the nucleus.
They are protons neutrons and electrons. Atomic Structures of Some Elements Hydrogen. To give an account or representation of in words.
The protons are placed in the nucleus of the atom while the electrons are situated in the orbital shell of the atom. An atom consists of three sub atomic particles. The density of ethanol is 0789 gmL.
These are found in the nucleus of the atom. It is composed of protons and neutrons. Describe the structure of a typical atom.
They are protons neutrons and electrons. ___ are negatively charged particles within an atom. Everything in the world is made out of atoms.
The ___ of an element equals the sum of the number of protons and neutrons in one atom of that element. The mass of an ___ is approximately 11837 amu. An atom is the basic unit of a chemical element.
Composition of the Atom. 702 describe the structure of an atom in terms of protons neutrons and electrons and use symbols such as 146C to describe particular nuclei. Protons and neutrons are in the nucleus electrons are in the shells.
The atom consists of a tiny nucleus surrounded by moving electrons. Protons have a positive charge. Add your answer and earn points.
Atoms are made of three main parts. Atoms are the basic components that a chemical element is made up of. Allie619 allie619 09222018 Chemistry Middle School answered Describe the structure of an atom 1 See answer Advertisement Advertisement allie619 is waiting for your help.
Carbon is described as The chemical element of atomic number 6 a nonmetal that has two main forms diamond and graphite occurs in impure form in charcoal soot and coal and is present in all organic compounds What system is. An atom consists of three sub atomic particles. An atom has protons and electrons.
The atomic number and the mass number of. The nucleus contains all the protons and neutrons of the atom. The way these electrons are positioned is known as the electron configuration.
The most abundant isotope of hydrogen on the planet Earth is protium. Composition of the Atom The atom consists of a tiny nucleus surrounded by moving electrons. Describe the composition of the nucleus of an atom.
Though the word atom originally denoted a particle that cannot be cut into smaller particles in modern scientific usage the atom is composed of various subatomic particles. Discreet Italian police described it in a manner typically continental. Protons neutrons and electrons are the particles that make up atoms and are accountable for their mass and charge.
According to Bohrs theory. The nucleus which is in the heart of the atom and includes protons and. These subatomic particles include protons and neutrons which are located in the nucleus and electrons which are located in the cloud or field that surrounds the nucleus.
The middle of the atom is thr nucleus. Carbon has two stable isotopes 12C and 13C. Physics and chemistry the smallest component of an element having the chemical properties of the element.
D It is composed of protons neutrons and electrons. Of these isotopes 12C has an abundance of 989. Electrons have a negative charge.
Describe the structure of a typical atom. The constituent particles of an atom are the electron the proton and the neutron. ___ of an element have the same atomic number but different mass numbers.
C It is composed of protons and electrons. An atom is the tiniest unit of matter that preserves all of an elements chemical properties. The protons positive are inside of the atom while the electrons negative are on the outside.
Calculate the inner diameter of the tube in centimeters. The nucleus contains protons which have a positive charge equal in magnitude to the electrons negative charge. Atoms join together to form molecules which interact to form solids gases and liquids.
The answer to your question is. B It is composed of protons and neutrons. Each shell can hold a certain amount of electrons before moving onto the next shell.
The nucleus contains protons which have a positive charge equal in magnitude to the electrons negative charge. The atom consists of a small positively charged nucleus at its centre and surrounded by negatively charged electrons in a definite circular path. They obtain subatomic particles in their nucleus and in the electron cloud that surrounds the nucleus.
These are found in the nucleus of the atom. The mass of ethanol needed to fill the tube is found to be 4523 g. Atoms are made up of protons neutrons and electrons.
An atom contains protons neutrons and electrons. The nucleus may also contain neutrons which have virtually the same mass but no charge. Neutrons have a neutral charge or no charge.

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets

Atom Infographic Infographic Poster Infographic Infographic Design

Comments
Post a Comment